
|
Fuel
Function
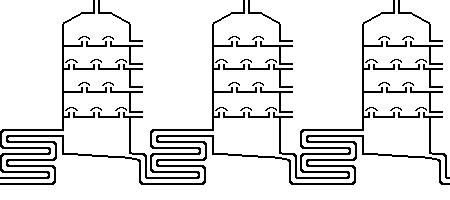
Distillation separates the single components of a substance according to their different boiling points. Taking for example the left distillation tower, the gaseous components will settle completely on top, which are
later on, for example used in gas cartouches or for the production of liquefied petroleum gas. Subsequently, row after row, there are the fuels for petrol engines, jet planes
(kerosene) and diesel engines. The next stage delivers besides different mineral oils also, e.g., candle wax and fuels for ships (outside the harbour). With the rest streets and house roofs are tarred. These were only
the products close to the vehicle technology. Of course, the chemical and plastic-processing industry is another large consumer of crude oil products.
How it works
Crude oil is basically not a uniform substance with one solid boiling point. It consists of different hydrocarbons. Engine oil is sometimes referred to as 'fully synthetical'. This sounds as if fully synthetical oil would only
consist of artificially made substances. In reality a lot of additives on the basis of hydrocarbons are present which descend, again, from crude oil. The figure on top shows three distillation equipments. The first two
differ by temperature (350 and 500°C), while the third besides the higher temperature adds depression (vacuum distillation). Nevertheless, this separation does not produce enough of the urgently demanded and,
therefore, well paid fuel. Therefore, a thermal and/or catalytic crack process is added afterwards for the components with a relatively late boiling point, to raise the fuel portion. During this process, long chained
alkanes are torn apart, forming short chained alkanes. Less valuable crude oil components become petrol.
If cracked components are added, the octane rating raises because the antiknock properties improve. This is another important step in the after treatment. During the platinum re-grouping chain molecules are also
split and connected to new branched molecules. Regrouping raises the portion of premium fuel. For diesel oil a very high cetane number is important.
This is not at all a complete
summary of treatment possibilities. Especially important are the almost entire desulphurisation and additives to prevent, e.g., deposits. The fuel production is more and more important for fuel consumption and the
exhaust gas quality. Besides, the margin for the viscosity and the fuel distillation range summer/winter becomes smaller and smaller. Some engineers state that the fuel manufacturers can contribute more to the
solution of the environmental problems than the vehicle manufacturers, 'synthetic' fuel will be our future.
|
|