Hydrocarbon
Function
If hydrocarbons are transformed into petrol, especially the antiknock properties are important. During the production of diesel oil, however, ignitability is
important.
How it works
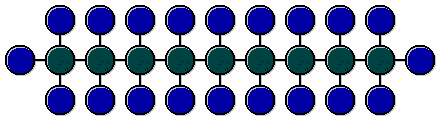
Hydrocarbons (e.g., H16C11 form the bases of fuels and lubricants. Their behaviour is decisively determined by the molecule construction. The quadrivalent carbon can establish a
connection with two neighbouring carbon atoms and two hydrogen atoms. This results in a simple carbon (green in the figure) and hydrogen (blue in the figure) molecule chain. These chains without network are nice
because of their ignitability as a basis for the diesel production, but almost useless for the production of petrol.
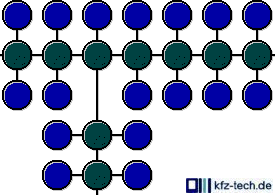
Nevertheless, instead of linking with a hydrogen atom the carbon can also establish a link with another carbon atom and therewith add another chain. This forming of larger branched molecule chains is stimulated in
the refinery by the influence of catalytic converters (polymerisation) and produces petrol with good antiknock properties.
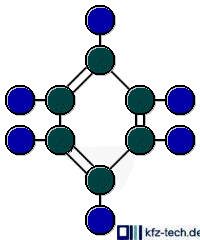
If the hydrocarbons contain less short chains and instead more ring-shaped molecules, the antiknock properties are also raised. The portion of such 'aromatics' is especially high with premium petrol.
|