 Chemistry - Metals 4 Chemistry - Metals 4
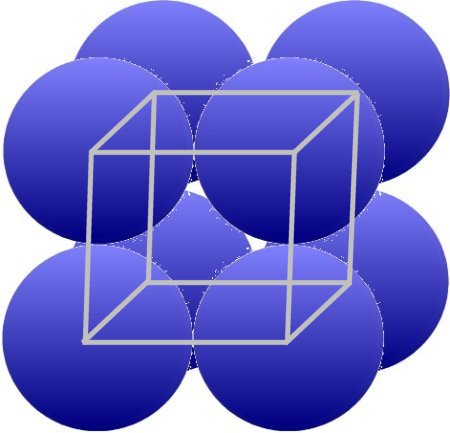
It is not always enough to depict the structure of metals, as can be seen in the chapter Metals 3. A real lattice can only be created if the centers of the individual particles are connected by lines. This results in metal distances of 0.25 - 0.5
nm (0,000 000 000 25 - 0,000 000 000 5 m).
In addition to the various crystallization nuclei already mentioned, there is a wealth of deviations and disorder in the real structure, which also increase with temperature or e.g. with plastic deformation. They are divided into lattice sites
not occupied or occupied by foreign atoms or additionally created.
However, we do not want to spend too much time on the structural analysis of grids, but rather simply look at the normal cooling process of iron (Fe) and the grids that occur during it. We do this here without the often associated carbon.
Let's look at the cooling from 1.536°C (δ-Fe).
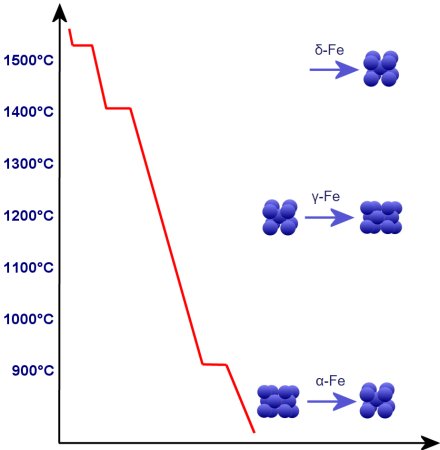
Here the Fe begins to organize itself in the form of crystals with cubic-space-centered grids. From 1,401°C a higher density is created by a cubic surface-centered arrangement (γ-Fe). From 911°C
it then transforms into cubic space-centered form (α-Fe). Between 1.401°C and 911°C δ-Fe becomes γ-Fe and from 911°C γ-Fe becomes α-Fe.
However, the center distance of the atoms becomes larger from δ- to γ-Fe and even smaller to α-Fe than with γ-Fe, i.e. altogether the smaller
packing density can be more than compensated. From 911°C the conversion is directed to α-Fe, the proportion of which increases.
We have omitted the so-called Curie temperature of 769°C here because, although it affects the magnetic properties of iron, it does not affect its crystallization, as one would expect only from the
found out later. For this temperature up to 911°C the name β-Fe was originally intended.
We will take a closer look at the γ-Fe (Austenite) and α-Fe (Ferrite). The former has favourable properties for hot forming, e.g. forging. Here, however, we leave the pure iron
and we deal with the carbon and its possible deposits in the iron crystal lattice.
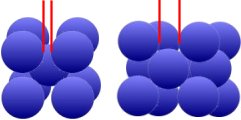
As you can see from the picture above, there is more space between the iron atoms in the cubic surface-centered lattice of γ-Fe (Austenite) on the right than in the cubic space-centered lattice of α-Fe (Ferrite). The
temperature above 911°C thus favors the storage of carbon atoms.
|