|
Battery
| Acid density: 1,12 g/cm³ | Cell voltage: < 2 V |
| Acid density: 1,28 g/cm³ | Cell voltage: 2,4 V |
Combining high efficiency and low weight, the battery should deliver the electric energy stored by the running of the engine, primarily
to enable quick starting. In
technical terms, it is actually called a collector (accumulator) which explains its job fairly appropriately.
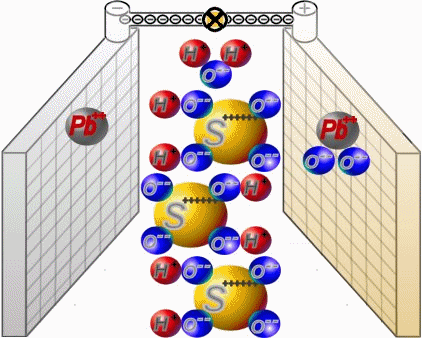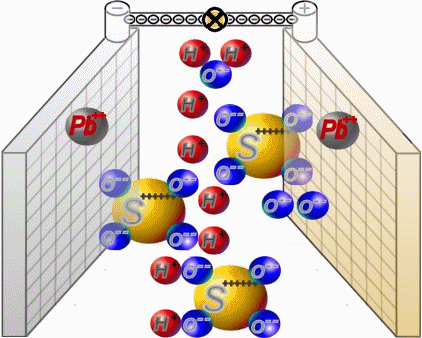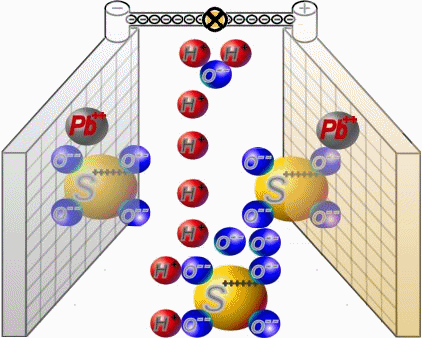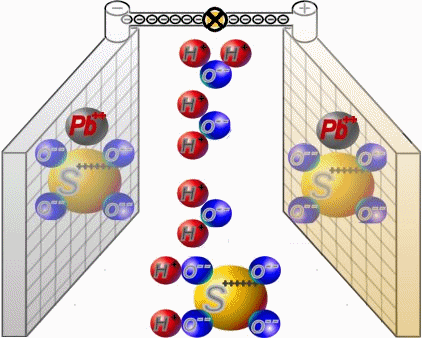
Discharge
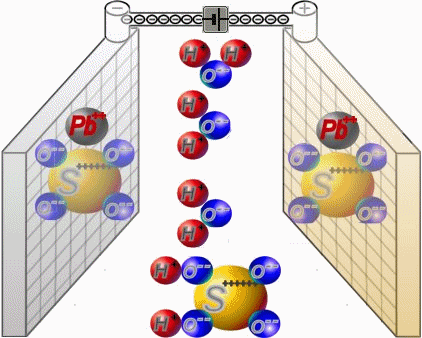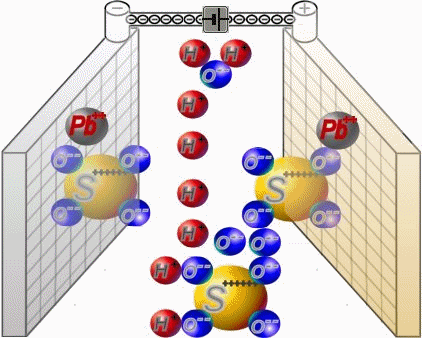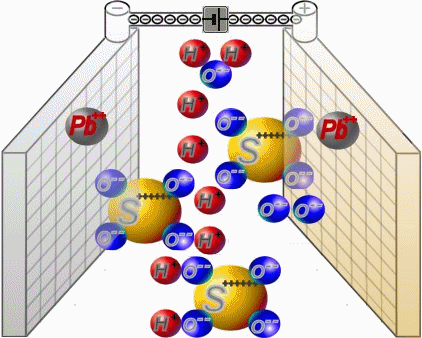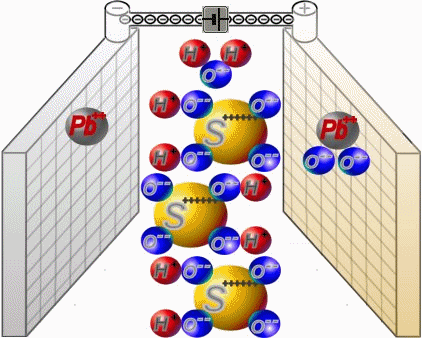
Two sets of plates per cell are arranged in a charged battery such, that in each case a positive plate with brown lead-dioxide is followed by anegative plate with grey lead. Between them, synthetic separators
preventany direct contact. Each cell is filled with diluted sulphuric acid.
When discharging, the sulphate of the sulphuric acid combines with the lead of the plates. Water is formed from the hydrogen of the sulphuric acidand the oxygen of the positive plates. In a completely discharged
battery, both plate sets contain lead sulphate. What's left is almost only water.
Charge
When charging, the chemical processes run off in the reverse order. Because the sulphuric acid portion raises the density of the liquid, one can recognise through this, the condition of the charged cell. In 12V-
batteries, six cells are switched one after the other. Theoretically it can reach up to 16.5 V, nevertheless, in the vehicle it is charged with approx. 14 V.
Apart from the tension and the maximum current, the capacity is an important measure of the size of a battery. One can recognise the condition of the charged battery by the density of the electrolyte. Because
sulphuricacid is heavier than water (1 g/cm³), 1.28 g/cm³ indicates a higher sulphuric acid portion, and less than 1.1/cm³, a higher water portion.
| If one is thicker, then the positive terminal. |
By charging too quickly, and/or, at more than 80 percent charge, detonating gas can develop. Therefore, the charging process should ideally, beslow, perhaps even interrupted, and/or the room should be well
ventilated. The formation of oxyhydrogen is mistakenly referred to as 'cooking', because here are developing small blisters similar to the water. Here, the heating is not as dangerous as the possible ignition of the
oxyhydrogen gas, which consists of water and oxygen. 8/11
Because the positive plates tend to deform rather, they are sometimes surrounded by negative plates
(-> odd number of plates per cell). |
|
|